Using the "Black Box" Approach to Enliven Introductory Physics Labs
Joe Amato and Colleagues
I. Introduction
It is well established that when undergraduates are exposed to research or research-like activities, they are strongly motivated to continue their studies in physics or astronomy. At many schools, juniors and seniors have ample opportunity to participate meaningfully in publishable research, often in addition to enrolling in advanced lab courses which expose students to a research-like setting. But attrition is most serious among beginning students, and so it is vitally important to inspire and encourage students who are enrolled in our challenging introductory courses. One way to do this is to replace the lab exercises that are customarily used in our introductory courses with ones that, in some sense, expose beginning students to the spirit and challenge of scientific research.
This approach was suggested in 1988 by Anthony French [1], who deplored the typical lab exercise as tending to "reinforce this picture of physics as a cut-and-dried, finished, essentially dead subject. Experiments to verify that momentum is approximately conserved, or that g has the value that every student already knows it to have, are scarcely calculated to inspire curiosity about the way the world works." He then proposed an alternative approach: "I believe that much could be done to reformulate some of our simple laboratory exercises so that they become genuine questions with the answer not known to the student (or, perhaps, to anyone) in advance..." We have adopted this "black box" approach at Colgate, and believe it to be an unqualified success. In the following sections, we present some examples of "black box" labs that are presently employed in our introductory physics sequence.
II. Black Box Labs for Introductory Mechanics
It can be exceedingly simple and inexpensive to modify existing labs, or develop new ones, to incorporate the black box approach. For example, one of the first labs our students encounter in the calculus-based mechanics course addresses measurement uncertainty and its propagation. Working in pairs, students are given a brass cylinder (each cylinder differs from the others, with length and diameter ≈ 2 cm) that has been asymmetrically etched in acid so that its dimensions vary roughly by ± 0.01 cm. Treating this variation as uncertainty, they are asked to determine the cylinder’s volume V ± ΔV. The instructor then measures its mass, using a digital scale, and the students then calculate the cylinder’s density. The "black box" aspect comes next: students are given a second cylinder made from the same material (brass), and are asked to determine its volume and predict its mass M ±ΔM. When they are satisfied with their calculations, they present their second cylinder to the instructor for weighing. Their grade depends on agreement between their calculated mass and the measurement. If they agree, and their uncertainty calculations are correct, they receive an "A." If they do not agree – on the first try – they receive a lower grade. The moment of measurement is filled with trepidation, followed (usually) by elation, but sometimes by disappointment. It is both exciting and educational.
This approach to lab work is invariably effective: students are presented with a single well-defined task, and it is their responsibility to complete it successfully. (Nearly all groups are successful.) Although there is no concealed component involved, the mass of the second cylinder is unknown a priori, so that careful measurements and calculations are clearly essential and need no further motivation.
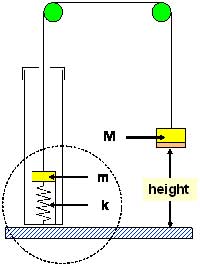
Figure 1
In the example shown, the hidden contents consist of a cylindrical disk of mass m attached to a fixed base plate by a spring of stiffness k. The disk is seated within a short plastic cylinder so that, at the lowest position of the mass, the spring is still stretched by a distance x0. This arrangement is carefully described to the students, but they must find a way to determine values for m and k. Briefly, the spring stiffness is determined by the slope of h(M), and the unknown mass m is then found by measuring the period of oscillation of the apparatus (M+m).
In a more challenging follow-on experiment, the mass-spring apparatus is removed from the PVC tube, which is then filled half way with water. A slender polystyrene rod (2.5 cm diameter, 30 cm long) is attached to the string and lowered into the water. No description of the tube’s contents are provided to students, who are asked to construct a physical model of the contents that is consistent with their h(M) data. This data (Figure 2) is more complicated than in the above case: only when the rod is partially submerged (between Min and Mout on Figure 2) does the height vary linearly with mass M. Indeed, because the specific gravity of polystyrene is 1.05, the force needed to extract the rod from the water varies from near-zero to the full weight of the rod, mimicking a spring force convincingly. The abrupt changes in behavior at Min and Mout cannot be ignored, however, and it is a challenging assignment to explain the entire data set. In this exercise, we allow student groups to collaborate, and give special "recognition" to the first group to "publish" correct results. Of course, there is more than one model that is consistent with the data (i.e., two masses attached with a spring and length of string), and we award full credit for models that "work" correctly. In many ways, this is akin to how real research is conducted.
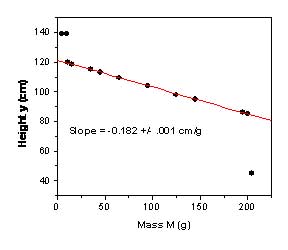
Figure 2
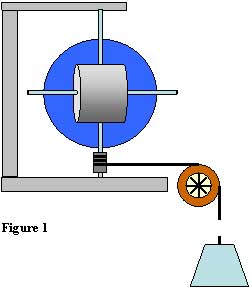
The "hidden object" strategy is limited only by the instructor’s imagination, and can be employed to exercise students’ understanding of a broad variety of physical concepts. For our final lab experiment in Mechanics, a simply-shaped object such as a cylinder, disk, or square bar is suspended by lightweight axles that pass through the object’s center of mass along its principal axes of rotation. The object is concealed within a thin opaque spherical shell with the axles protruding (Figure 3) so that they can be inserted into low friction bearings for spinning the object. String is wound around the lower bearing, and passes over a pulley to a hanging mass. When the mass is released, the rotational acceleration of the object is recorded and used to find the moment of inertia of the assembly about each of the three axes of rotation. These data, along with the mass of the object, are used to determine the size and shape of the object. This is a challenging project, and requires careful measurements and error-free calculations. Each student works on a unique object, so that collaboration is not possible. Ideally, even the instructor does not know what is inside a particular shell.
At the GRC, we demonstrated a much simpler and less expensive version of this apparatus. Rather than spinning the object, it is hung from a stainless steel torsion wire and its period of oscillation is found. The torsion wire is calibrated using a known (visible) object such as a disk. With careful measurements, the dimensions of the hidden object can be determined to within 1 %. Unambiguous results are easily obtained, and the exercise can be very satisfying for students. We are careful to point out that this exercise is akin to, say, a nuclear physics experiment wherein the shape of a nucleus is determined by studying its rotational properties.
III. Black Box Labs for Modern Physics
The black box strategy, of course, is not limited to mechanics. Although we have not yet done so, it is easy to imagine black box exercises in electromagnetism, fluids, and thermodynamics. We have, however, designed and deployed two black box experiments for modern physics. In the first experiment [2], a glass bell jar containing a pure gas such as N2 is evacuated through a tiny orifice (diameter = 400 μm) by an inexpensive homemade sorption pump. By measuring the gas pressure vs. time during the evacuation period (about 15 minutes), the average speed of the gas molecules (≈ 500 m/s) can be determined to within a few percent. Depending on the context of the experiment, this can be the goal of the exercise; better yet, students can be asked to use their calculated speed to identify the gas. The second experiment [3] employs a novel microwave Bragg scattering apparatus, using a rotating "crystal" array of metal rods, to mimic x-ray diffraction and discover the meaning of Miller indices. The method yields data that are sufficiently accurate so that the spacing and orientation of a shrouded (unknown) crystal can be determined easily. The apparatus for these two experiments are somewhat more complicated than that described for the mechanics labs, and we refer the reader to the published accounts for full details.
IV. Summary
Black box labs are an effective way to instill the spirit of research into the introductory curriculum. Properly designed, they can "inspire curiosity about the way the world works." They can be easy and inexpensive to implement, challenging and fun for students, and enjoyable to teach as well. They are suitable for reinforcing many, but not all, topics encountered in the introductory curriculum. In 1989, Alfred Romer [4] pointed out that lab exercises and apparatus are constrained by available time, relevance to the curriculum, significance of the exercise, and the probability of student success. Used with discretion, the black box strategy can be a valuable way to enhance and enliven the introductory physics curriculum.
V. Bibliography
1. A. P. French, Some thoughts on introductory physics courses, Am. J. Phys. 56 110 (1988)
2. J. C. Amato and R. E. Williams, An inexpensive gas effusion apparatus for the introductory laboratory, Am. J. Phys. 59, 346 (1991)
3. J. C. Amato and R. E. Williams, Rotating Crystal Microwave Bragg Diffraction Apparatus, Am. J. Phys. 77 942 (2009)
4. A. Romer, private communication
Joe Amato is a Professor of Physics Emeritus at Colgate University. Throughout his career he has developed a number of innovative laboratories for use in undergraduate physics laboratories. His research interests are in low-temperature physics, condensed matter physics and granular materials.
Disclaimer- The articles and opinion pieces found in this issue of the APS Forum on Education Newsletter are not peer refereed and represent solely the views of the authors and not necessarily the views of the APS.
