Reflections on the Centenary of Lord Rayleigh’s Death
By John Meurig Thomas and Edward A Davis, Department of Materials Science and Metallurgy, University of Cambridge

Painting of the Third Baron Rayleigh by Sir Philip Burne-Jones
Introduction
Even when judged by the highest standards of intellectual achievement, the legacy of the Third Baron Rayleigh (John William Strutt 1842-1919) ranks among the very highest in the entire realm of the physical sciences. The list of eponymous phenomena and equations associated with him (Figure 1) reflects his prodigious versatility and pre-eminence as both a theoretician and experimentalist. His enduring influence on a vast range of present-day scientific and technological endeavours requires no elaboration. Suffice it to recall that Rayleigh waves are of vital importance in seismology, his mathematical procedures are still used by acoustic engineers and quantum theorists, the Rayleigh criterion limits the performance of optical instruments, and it is appropriate to recall also that he was the first to explain theoretically why light from the sky is blue and polarized, and sunsets are red.
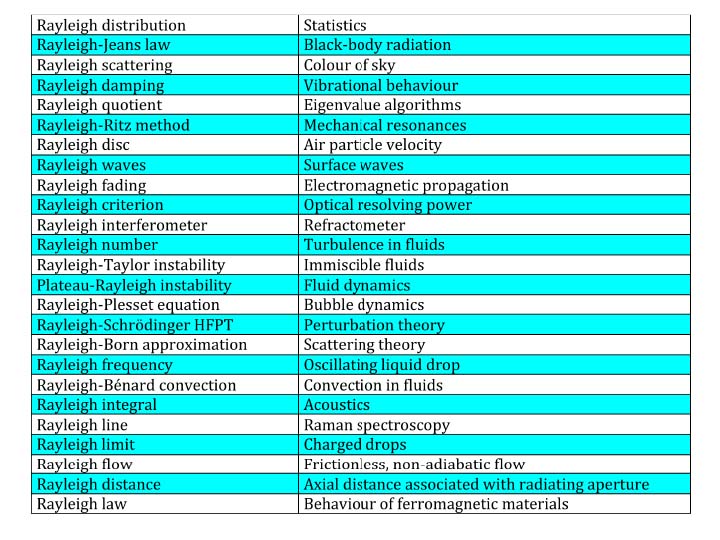
Figure 1. Compilation of effects, laws, mathematical methods, etc. named after Lord Rayleigh
Rayleigh died in the summer of 1919 at his Baronial home in Terling, Essex (Figure 2), where he carried out most of his work [1]. To commemorate the centenary of his death, the present (sixth) Lord Rayleigh and his family held an ‘Open House’ on May 11th 2019, which was attended by many eminent physicists, engineers, chemists, geologists and historians of science, who were invited to tour Rayleigh’s still extant laboratories (Figure 3), his workshops and his study, to see the visitors book as well as many other items, including some of his extensive correspondence in the family archive. Amongst the large collection of letters, equipment, experimental rigs, photographs and specimens are items donated to him by his contemporaries, for example William Crookes (1832-1919), George Stokes (1819-1903), Joseph Larmor (1857-1942), J.J. Thomson (1856-1940), Lord Kelvin (1824-1907), Henry Rowland (1848-1901), and Nicholas Tesla (1856-1943).

Figure 2. Terling Place; home of the Strutt family. The laboratories created by the Third Baron Rayleigh are on two floors in the near-most wing of the house.

Figure 3. View of one of the rooms of the Third Baron Rayleigh’s laboratories.
The visitors’ book alone makes fascinating reading. Apart from the entry for May 1904 (Figure 4) when Rayleigh brought Kelvin and Rutherford together (as a result of their conflicting views of the age of the Earth [2]), there is the July 1908 list of guests that includes Lord Rayleigh’s brother-in-law, the Prime Minister A.J. Balfour, and the future Prime Minister H. H. Asquith with his wife and the 25 year old lady with whom he later became besotted, Venetia Stanley [3]. Eminent scientists whose names also appear in the visitors’ book include Albert Michelson, Edward Morley, Alfred Mayer, Hermann von Helmholtz, Alfred Cornu, Oliver Lodge, J J Thomson, William Thomson, G G Stokes, John Tyndall and William Crookes.

Figure 4. Extract from visitors’ book at Terling Place, May 1904 confirming meeting between Lord Kevin, Lord Rutherford and Professor Schuster.
Rayleigh’s network of contacts was enormous, partly because he held so many important posts: Cavendish Professor and Chancellor of the University of Cambridge (1879-1884), President of The Royal Society (1905-1908), progenitor of the National Physical Laboratory Teddington and Scientific Advisor to Trinity House quite apart from his closeness to another Prime Minister, Lord Salisbury, who was the uncle of his wife, Evelyn Balfour. Furthermore, his correspondence with scientists abroad, including Mendeleev, Nernst and Helmholtz, was considerable.
A great deal has been published about the life and legacy of Lord Rayleigh [4-8], but new information came to light at the Open House which has not hitherto been reported, and which adds to the esteem with which Rayleigh is held.
One of us (EAD) elaborated in an illustrated presentation on the breakdown of Rayleigh’s publications (Figure 5), all 445 of them [9], and the other (JMT) traced his persistence, initiated by the letter that was published in Nature in 1892, when he was pre-occupied with determining the density of nitrogen (Figure 6). This was to lead to the discovery of argon, which earned Rayleigh the 1904 Nobel Prize in Physics – the first to be awarded to a British physicist. His discovery of argon led, in turn, to the monumental paper that he published with Sir William Ramsey (the Nobel Prize winner in Chemistry in 1904) entitled Argon, a New Constituent of the Atmosphere [10].
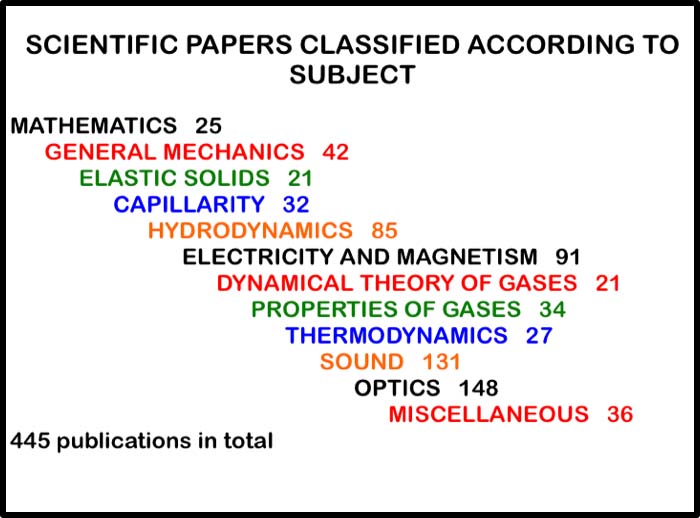
Figure 5. Breakdown of the Third Baron Rayleigh’s publications into topics.
An Outline of His Early Career
J. W. Strutt was Senior Wrangler, the top graduate in mathematics at Cambridge in 1865. He had been tutored, like other scientific luminaries in Cambridge, for example J.J. Thomson, J.A. Larmor, G. Darwin and A.N. Whitehead, by one of the most gifted tutors, E.J. Routh, who, in a 33-year career, coached over 600 pupils and guided 28 of them to be Senior Wranglers.
Writing almost 60 years after Strutt’s graduation, Sir James Jeans disclosed that “… there still lingers in Cambridge a tradition as to the lucidity and literary finish of his answers in the examination …”. The fine sense of literary style that Strutt exhibited, even under pressure in the examinations, never deserted him. Every paper he wrote, even those dealing with the most abstruse subjects is a model of clarity and simplicity and conveys the impression of having been written with effortless ease – see for example the opening sentence of his eighth paper “On the Light from the Sky” (figure 6).

Figure 6. Hand-written manuscript of Rayleigh’s classic paper explaining why the sky is blue.
Shortly after he graduated, the young Strutt struck up a friendship with the taciturn George Gabriel Stokes (1819-1903), another Senior Wrangler, who had become Lucasian Professor, and who, like another earlier occupant of that illustrious chair, Isaac Newton, was, in addition to being a redoubtable mathematician, also an accomplished experimentalist [11]. It was this latter quality of Stokes’, as much as anything else, that was responsible for Strutt’s affinity towards him.
In 1861, shortly after he was appointed as a Fellow of Trinity College, Strutt felt dissatisfied that there was no course available in the University of Cambridge at that time that would enable him to acquire experimental skills desirable for someone eager to pursue natural philosophy. Whilst Stokes gave him some encouragement and guidance in this regard, Strutt resorted to seeking help from G.D. Liveing, the then Professor of Chemistry at Cambridge. He received from this source, for six months or so, a course on analytical chemistry, which greatly assisted him in developing experimental skills. Indeed, he was to become an adroit experimentalist in addition to blossoming as a fiendishly able theoretician.
Rayleigh the Physicist
Rayleigh wrote the majority of volume 1 of his classic text ‘The Theory of Sound’ in 1872 while cruising on the Nile – a trip that had been prescribed as a recuperative measure following a serious attack of rheumatic fever. It contains detailed descriptions of the mechanics of vibrating systems, while acoustic wave propagation is treated in volume 2. Both texts are still in use today by acousticians. Such is the generality of many of Rayleigh’s mathematical descriptions of vibrations and waves that many of these could be applied to, or analogies found in, other fields - for example hydrodynamics, optics and electromagnetism - as indeed he demonstrated over much of his scientific life.
From an early age Strutt (as he then was) made himself an accomplished photographer, constructing pin-hole cameras and developing his own photographs. He used his expertise in this regard to fabricate diffraction gratings by contact printing and produced optical zone plates with light focusing properties. He established formulae for the resolving power of gratings, determined the criterion for the ultimate optical resolution achievable by optical instruments and contributed to an early understanding of the behaviour of spectroscopes. It was during this phase of his work that Rayleigh struck up correspondence with H.A. Rowland in Baltimore.
An extremely productive period of his life was during his tenure as Cavendish Professor from 1879 to 1885. His work there on electrical standards, in which he was assisted by his sister-in-law, Eleanor Sidgwick (née Balfour), provided new values for the units of resistance, current and voltage, which stood for many decades. No doubt these studies also influenced his later support for the establishment of the National Physical Laboratory in Teddington. His other interests were not neglected during this period; for example, he developed the Rayleigh disc for measuring the intensity of sound and introduced the concept of surface waves in solids, which are important in seismology as already mentioned but also in today’s electronic circuits as filters and delay lines. In total, 60 of his papers were published in this period, during which he also introduced practical classes as an important part of undergraduate teaching.
On his return to Terling, Rayleigh continued to work on a wide variety of problems, including wave propagation in periodic systems, stimulated by his observations of the colours of thin films, beetles, butterfly wings, etc. Magnetic phenomena, electrodynamics, viscosity, elasticity, wave-guide theory, optics and, of course, his first-love, acoustics, all figure in his numerous later publications. The story of the discovery of argon and his work in parallel with Ramsay to isolate the gas has been well documented [4,10,12]. What has recently come to light [13] is a ‘pli cacheté’ (secret packet) deposited by Ramsay with the French Académie des Sciences in July 1894 and first opened as recently as 2004. Deposition of such packets (sealed with wax) allowed an individual to lay claim to a discovery without actually publishing it. A translation (by Alwyn Davies [14]) of the document signed by Ramsay is as follows:
Guided by the experiments of Lord Rayleigh on the density of nitrogen, which show that the nitrogen obtained from the air by means of copper metal is more dense by one part in 231 than the nitrogen obtained by chemical means from ammonia, or from nitrous acid, I have studied the residue after a large quantity of nitrogen from air has been absorbed by means of magnesium at red heat. …….
….. I hope to decide soon if the gas is a modification of nitrogen, perhaps N3, or a new element, by carrying out the absorption by magnesium, making each experiment more quantitative by volume, and by measuring the ammonia or a combination of X with hydrogen, I can decide what is its approximate atomic weight. If it happens that I have a new gaseous element, I intend to name it Eikazote, with the symbol Ez.
To end this note, it should be recognized that it is to Lord Rayleigh, with whom I have been in communication, that this discovery is credited; he has shown the place to explore; I found the means to isolate this novel gas.
In fact, both Rayleigh and Ramsay worked independently to isolate the gas and both succeeded within a month of the packet being deposited (Figure 7). A joint paper and two Nobel Prizes followed, not to mention a whole new column in the Periodic Table!

Figure 7. The opening sentences of the letters in which Rayleigh and Ramsay report their simultaneous isolation of argon. (from reference [12]).
This section of the paper cannot be concluded without mention of the Rayleigh-Jeans law. Rayleigh’s work embraced essentially all aspects of classical physics known at the time. His theoretical treatment of the spectrum of black-body radiation accounted exceedingly well for the spectral distribution of energies at long wavelengths but, being classical, failed at short wavelengths - the so-called ultraviolet catastrophe. Although he was still very active at the age of 58 when Planck produced his formula and quantum concepts came to the fore to account for this evident failure, Rayleigh was rather reluctant to embrace these ‘new’ ideas and preferred to leave them to future researchers.
Rayleigh the Chemist
It is noteworthy that Rayleigh assembled in the 1870s one of the best-equipped chemical-physical laboratories in England. The only one that surpassed it in the range of chemicals and equipment had been established in the early 1800s, first by Humphrey Davy and then enlarged by Michael Faraday, at the Royal Institution in London [15]. Rayleigh’s laboratory, even to this day, is impressive. It is equipped with an unusually large collection of chemicals – far greater than was typical in a British university chemical laboratory fifty years ago.
Rayleigh’s skills as a practical chemist are reflected in the many papers he wrote, having carried out the experimental work himself. Thus, in his 1902 paper on the ‘Distillation of Binary Mixtures’ [16] he describes his detailed work on mixtures of alcohol, ammonia, and several acids with water, preceded by a theoretical outline of the phenomenon of distillation of pure liquids and liquids that form homogeneous mixtures. Other papers, such as his work on the electrochemical equivalent of silver [17] and periodic precipitation of crystals [18] (one of his last publications), testify to his skills as a laboratory-oriented chemist. Moreover, in connection with his discovery of argon – which followed from his finding that ‘pure’ nitrogen derived from the atmosphere was always slightly heavier than nitrogen he prepared from chemical sources, such as ammonia and urea – he devised several ingenious chemical methods of eliminating traces of oxygen from atmospheric nitrogen [12,19].
Rayleigh and Dimensional Analysis
Rayleigh’s landmark paper [20] where he established that the intensity of scattered light is inversely related to the fourth power of the wavelength used the so-called method of dimensional analysis. He also derived this relationship and the facts pertaining to the polarization of scattered light by rigorous mathematical analysis. Throughout his life he commended the virtue of using the method of dimensional analysis. Indeed, forty-five years after his landmark paper he wrote an article in Nature, under the title ‘The Principle of Similitude’ [21], which began with the following remark: “I have often been impressed by the scanty attention paid even by original workers in physics to the great principle of similitude”. In this article, Rayleigh bemoans the fact that this was not used as extensively as it should be. To press home this point, he recites fifteen examples of how information of vital importance can be readily retrieved by dimensional analysis. Here are just three:
- The velocity of propagation of periodic waves on the surface of deep water is as the square root of the wavelength,
- The resolving power of an object-glass, measured by the reciprocal of the angle with which it can deal, is directly as the diameter and inversely as the wavelength of the light,
- In a gaseous medium, of which the particles repel one another with a force inversely as the n’th power of the distance, the viscosity is as (n + 3)/(2n – 2,) power of the absolute temperature. Thus, if n = 5, the viscosity is proportional to temperature.
Concluding Remarks
The impression left on all the visitors to the ‘Open House’ celebration was that they were exposed afresh not only to a supreme theorist and experimentalist, but also to an individual who had an unquenchable curiosity for an enormous range of natural phenomena and their scientific interpretation. The soaring of birds [22], the sailing flight of the albatross [23] and the fascination of iridescent crystals [24] were topics that engaged his interest in the 1900s, and one of his last papers published in the year of his death was entitled On the optical character of some brilliant animal colours [25]. Few practitioners and historians of science in its broadest aspects would dispute that Lord Rayleigh was one of the most remarkable physical scientists of his generation.
Notes and References
- Contrary to the information given in Wikipedia, Rayleigh did not spend his whole scientific life in the University of Cambridge. He was the second occupant of the Cavendish Chair of Experimental Physics (after Maxwell died) but stipulated that he would hold this post for only five years (1879-1884). He later was engaged as Professor of Natural Philosophy at the Royal Institution, where, in 1898, he became joint Director with Sir James Dewar, of the Davy-Faraday Research Laboratory. For the rest of his life he worked in his own, privately-built laboratory in converted stables at the family seat at Terling.
- Rutherford gave a Friday Evening Discourse at the Royal Institution in early 1904, during the course of which he declared that the age of the Earth, based on his work on radioactivity, could be as large as a thousand million years in blatant contradiction to the estimates made by Lord Kelvin, who was in the audience. After the Discourse, Rayleigh told Kelvin that Rutherford was likely to be nearer the truth than Kelvin. He said he would arrange for the two to meet in Terling; the visitors book records when exactly they met and who the others were that attended.
- H.H. Asquith, the 54-year-old Prime Minister of the day became intensely associated with the 25-year-old Venetia Stanley who was present, along with Asquith and his wife and A.J. Balfour at Terling Place in July 1908. The definitive biography of Asquith (by Roy Jenkins, published in 1964) disclosed that Asquith was besotted by Miss Stanley and wrote frequently to her when he chaired cabinet meetings, even when his country was at war.
- Life of John William Strutt, Third Baron Rayleigh by Robert John Strutt (Fourth Baron Rayleigh) University of Wisconsin Press 1968
- Lord Rayleigh: The Man and His Work* by Robert Bruce Lindsay, Pergamon Press 1966
- J.H. Howard, Research of the Third Baron Rayleigh, Proc. of the Royal Institution, 60. (1988) p73.
- Maxwell’s Enduring Legacy; A Scientific History of the Cavendish Laboratory by Malcolm Longair, Cambridge University Press 2016, p69.
- E.A. Davis, Lord Rayleigh: His Works and Laboratories, in The Roots of Physics in Europe, Proceedings of the first European Symposium on the History of Physics, ed. P.M. Schuster, Living Edition Science, Austria 2010, p35.
- All but a few are single-author papers which Rayleigh submitted in long hand.
- Lord Rayleigh and Professor William Ramsay, Argon, a New Constituent of the Atmosphere, Phil Trans Roy Soc A, (1895) pp187-241.
- It was Stokes who discovered the phenomenon of fluorescence, which he demonstrated at a Friday Evening Discourse at the Royal Institution in 1853.
- J.M. Thomas, Argon and the Non-Inert Pair, Angewandte Chemie International Edition, 43 (2004) p6418.
- Yves Jeannin, Comptes Rendus Chimie, 8 (2009), pp3-7. The assertion of priority while avoiding publication is discussed in this case and more generally by Michael Jewess, Royal Society of Chemistry Historical Group Newsletter, No 71 (Winter 2017), 22-27 (hard copy), 12-14 (online).”
- Alwyn Davies, Royal Society of Chemistry Historical Group Newsletter No.70 (Summer 2016) pp33-37 (hard copy),pp18-20 (online).
- The Clarendon Laboratory was established in Oxford in 1868, the Cavendish Laboratory in Cambridge in 1872. Thanks to the efforts of Prince Albert, the Royal College of Chemistry in London (by 1907 indirectly subsumed into Imperial College) was established in 1845 and Prince Albert facilitated the appointment of one of Germany’s foremost experimentalists, A. F. Hofmann, to be the first Professor of Chemistry in the College. The Royal Institution had an impressive laboratory from 1801 onward.
- Lord Rayleigh, On the Distillation of Binary Mixtures, Phil. Mag. IV (1902) p521.
- Lord Rayleigh, The Electrochemical Equivalent of Silver, Nature, LXI (1897) p292.
- Lord Rayleigh, Periodic precipitations of crystals, Phil. Mag. XXXVIII (1919) p738.
- In a letter to Nature XLVI (1892) p512 he wrote:
‘I am much puzzled by some results on the density of nitrogen, and I shall be obliged if any of your chemical readers can offer suggestions as to the cause. According to the methods of preparation, I obtain two quite distinct values. The relative difference, amounting to about one part in 1000, is small in itself, but it lies entirely outside the errors of experiment, and can only be attributed to a variation of the character of the gas.’ - Lord Rayleigh, On the Light from the Sky, its Polarization and Colour, Phil. Mag. XLI (1871) pp 107-120, 274-279.
- Lord Rayleigh, The Principle of Similitude, Nature, XCV (1915) p66.
- Lord Rayleigh, Soaring of Birds, Nature, XXVII (1883), p534.
- Lord Rayleigh, The Sailing Flight of the Albatross, Nature, XI (1889) p14.
- Lord Rayleigh, On some Iridescent Films, Phil. Mag. XXIV (1912) p751.
- Lord Rayleigh, On the Optical Character of Some Brilliant Animal Colours, Phil. Mag. XXXVII (1919) p98.
The articles in this issue represent the views of their authors and are not necessarily those of the Forum or APS.
