A Tutorial on the Basic Physics of Climate Change
Articles
The following article has not undergone any scientific peer review, since that is not normal procedure for American Physical Society newsletters. The American Physical Society reaffirms the following position on climate change, adopted by its governing body, the APS Council, on November 18, 2007: "Emissions of greenhouse gases from human activities are changing the atmosphere in ways that affect the Earth's climate."
By David Hafemeister & Peter Schwartz
Abstract: In this paper, we have used several basic atmospheric–physics models to show that additional carbon dioxide will warm the surface of Earth. We also show that observed solar variations cannot account for observed global temperature increase.
The Intergovernmental Panel on Climate Change (IPCC) has projected a likely temperature rise of 3 oC (2 to 4.5 oC) from a doubled CO2 of 560 ppm in this century.[i] Many believe that a rise of 2–2.5 oC will cause a “dangerous anthropogenic interference with climate.” Earth has already had a rise of 0.8 oC in less than one-half century, and it is projected to rise another 0.6 oC as the planet adjusts to the present level of CO2. Scientists have accumulated compelling evidence besides the temperature data to document a warming Earth. The observations include the shrinking of the northern ice cap (40% thinner in 30 years, and a considerable loss in surface area in the last year) and Greenland’s glaciers, lakes are frozen a shortened time by about two weeks, and summer is two weeks longer as determined by animal and plant cycles. The discussion sensibly moves to two main questions: “Are non-anthropogenic causes of warming significant” and “how much warmer will Earth become?”
We will not review the scientific literature, as that path is well trod. Rather, we present some basic physics models, to shore up basic understandings. [ii] Put a blanket over a light bulb, and you will have a fire. For the full power of the light bulb to pass through the blanket, the inner temperature must rise considerably. The atmosphere is not a mere thermal resistor, but the analogy is illuminating. Svante Arrhenius, a Swedish physicist, first suggested in 1896 that increases in atmospheric CO2 would lead to global temperature rises. Below, we conduct an analysis in a similar fashion.
The naturally occurring greenhouse gases (present before industrialization) cause the earth to be 33 oC warmer than if there was no infrared trapping by the atmosphere. One can attribute 21 oC of that warming to the IR trapping of water vapor, 7 oC to CO2 and 5 oC to other gases. If we add even more CO2, we should expect it to increase the surface temperature. There are also feedbacks, but IPCC has observed that feedbacks are more positive than negative, meaning they will further increase warming. It is our belief that “theory leads experiment” on climate change because all well-accepted atmospheric models predict a temperature rise. The data over the past decade is now solidifying in general agreement with theory. General Circulation Models (GCM) and our basic models connect cause and effect. Some critics believe that the warming is “directly linked to two distinctly different aspects of solar dynamics: the short-term statistical fluctuations in the Sun’s irradiance and the longer–term solar cycles.” [iii] We will show that observed solar fluctuations cannot be responsible for the presently observed global climate changes.
The carbon released worldwide from burning carbon and deforestation has recently been about 7.1 Gt/yr. The number of CO2 molecules released is
NCO2 = (7.1 x 1015 g/yr)(6.02 x 1023/mole)/(12 g/mole) = 3.6 x 1038 molecules CO2/yr. (1)
The mass of the atmosphere is the surface area of Earth times the atmospheric pressure of 105 Pascal divided by g:
Matmos = PA/g = (105 Pa)(4p)(6.4 x 106m)2/(9.8 m/sec2) = 5.3 x 1018 kg. (2)
The number of O2and N2 molecules in the atmosphere is
Natmos = (5.3 x 1021 g)(6.02 x 1023/mole)/(29 g air/mole) = 1.1 x 1044 molecules. (3)
This gives the rate of increase in concentration of CO2 molecules,
cCO2 = NCO2/Natmos = (3.6 x 1038 CO2/yr)/(1.1 x 1044 air) = 3.3 ppm/yr. (4)
This is more than twice the atmospheric CO2 rise of 1.4 ppm/yr (325 ppm in 1970 to 354 ppm in 1990 to 370 ppm in 2000). Thus, about half of the CO2 remains in the atmosphere, the other half goes into sinks in the oceans and on land.
CO2 Before Industrialization. The pre-industrial CO2 level was 280 ppm in 1800. By 1959, the level had grown to 316 ppm. We can estimate total change in concentration by integrating backwards in time. Using a rate of 0.9 ppm/yr in 1959 and a global carbon rate growth rate of aboutl= 3%/year, the increase in CO2 concentration between 1800 and 1959 should be about
ΔcCO2 = 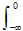
![]() (0.9) eltdt = 0.9(e0 – e
(0.9) eltdt = 0.9(e0 – e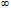
Subtracting this from the 1959 value of 316 ppm gives a pre-industrial CO2 level of 285 ppm, close to the accepted value of 280 ppm.
CO2 in the 21st Century. 2050 CO2 levels may be obtained by projecting 60 years growth onto the 1990 level of 354 ppm. Energy Information Agency estimated a business-as-usual approach will give 2%/yr global growth in fossil fuels, for a 2050 concentration of
cCO2 = 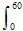
![]() (1.4 ppm/yr) e0.02t dt + 354 ppm = 162 ppm + 354 ppm = 516 ppm. (6)
(1.4 ppm/yr) e0.02t dt + 354 ppm = 162 ppm + 354 ppm = 516 ppm. (6)
This figure is consistent with most business-as-usual projections.
Upper-Atmospheric Temperature Ta.Earth's temperature is determined from a heat balance between absorbed energy from solar flux so = 1367 W/m2 and infrared emission to space. The solar power intercepted by the area of Earth's disk (pRE2so) is distributed over the entire spherical area (4pRE2), giving an average solar flux of so/4 = 1367/4 = 342 W/m2. Of this, 70% is absorbed by the Earth, and 30% is reflected (Earth’s albedoa = 0.3 in the visible), giving an average fluxabsorbed by surface and atmosphere,
sabsorbed = (1 – a)(so/4) = (1 – 0.3)(1367/4) = 239 W/m2. (7)
Absorption by clouds and atmosphere reduces solar flux at the surface to an average of about 200 W/m2. The energy absorbed by Earth’s surface is sent upward by infrared, evaporation and air currents, which is captured by the atmosphere or passes directly to space. In our first model, we assume that allthe absorbed energy is reradiated to space as IR from a thin surface at the top of the atmosphere.
The power balance at the top of the Earth's upper atmosphere is
Pin = (1 – a)(pRE2so) = Pout = esTa4(4pRE2), (8)
where temperature at the top of the atmosphere Ta is in Kelvin, s is the Stefan–Boltzmann constant, 5.67 x 10–8 W/m2K4, and e is emissivity (about 1 for 10–micron infrared). Solving for the upper atmosphere temperature,
Ta = [(1 – a)so/4es]1/4 = [239 W/m2/se]1/4 = 255 K = –18oC = 0oF. (9)
The temperature in the middle of the troposphere is 255 K at 5 km above the surface (and at 50 km.) This is 32 K colder than the observed average surface temperature of 287 K (14.0 oC with 1997 averages of 14.6oC in the northern hemisphere and 13.4oC in the southern hemisphere).
As a comparison we calculate Ta-V for Venus, which has a higher solar flux since the radius of its orbit is only 60% that of Earth:
so-V = so(rE/rV)2 = (1367 W/m2)(1.50 x 108 km/1.08 x 108 km)2 = 2610 W/m2. (10)
However, Venus's higher albedo of 0.76 reflects a greater fraction of sunlight, greatly reducing the average absorbed flux to
(1 – a)so-V/4 = (1 – 0.76)(2610 W/m2)/4 = 157 W/m2, (11)
which is smaller than Earth's 239 W/m2. The upper atmospheric temperature of hot Venus,
Ta-V = [157 W-m–2/s] 1/4 = 229 K (12)
is 26 K colder than Earth's 255 K. However, Venus's higher CO2 concentration traps IR, giving it a surface temperature of 750 K, three times Earth's surface temperature of 287 K.
Surface Temperature Ts. Our zero-dimensional box model did not take into account the following variable factors: Reflection, absorption and emission by air, aerosols, clouds and surface; Convection of sensible and latent (evaporation) heat; Coupling to oceans and ice; Variations in three dimensions; and Variable solar flux.
Next, we estimate the surface temperature Ts without considering Ta. We assume that all the solar flux that is not reflected is transmitted through the air and totally absorbed by the Earth's surface fabsorbed = (1 – a)so/4. The warmed surface radiates as a blackbody, and also loses heat through rising in air currents or evaporated moisture. We allow a fraction of the light radiated from the earth, fIR to be absorbed by the atmosphere, which is mostly in the infrared. The atmosphere radiates 50% of the IR absorbed flux to space and 50% to Earth, giving an IR flux downward of (fIR/2)fabsorbed. Again, a fraction fIR of this energy is absorbed in the atmosphere again and 50% of this radiates downward and is absorbed by the surface, (fIR/2)(fIR/2)fabsorbed. This process gives an infinite sum in the energy balance:
fabsorbed + (fIR/2)fabsorbed + (fIR/2)2fabsorbed + (fIR/2)3fabsorbed + ….. + (fIR/2)nfabsorbed = sTs4. (13)
After manipulation, this becomes
fabsorbed/[(1 – (fIR/2)] = sTs4. (14)
We obtain Earth’s surface temperature of Ts = 287 K with fIR = 0.76. For the extreme case of no IR absorption in the atmosphere (fIR = 0), we obtain Ts = 255 K, the temperature of the upper atmosphere. For the other extreme case of 100% IR absorption in the atmosphere (fIR = 1), we obtain Ts = 303 K, consistent with the next calculation.
Ta and Ts Together: Next, we assume that all sunlight is transmitted through the air and absorbed by the Earth's surface. We vary the one free parameter, the emissivity of the atmosphere ea, but retain the surface of Earth as a blackbody with eE = 1. Equation 15 balances heat flow in the single layer of air. The left side doubles the infrared flux emitted by Earth’s atmosphere, since IR goes both up into space and down to Earth's surface, essentially doubling the radiating surface area. This is balanced with IR flux emitted from Earth’s blackbody surface and absorbed by the gray body atmosphere.
2easoTa4 = easTs4 (15)
Equation 16 is an energybalance at Earth’s surface. The left side is the sum of solar energy absorbed at the surface and the absorbed downward flow of IR from the atmosphere, which is balanced with upward IR flux from the surface,
(1 – a)so/4 + easTa4 = sTs4. (16)
Solving equations 15 and 16 gives
Ts = 21/4Ta and sTs4 = (1 – a)so/4(1 – ea/2). (17)
If the air layer is a blackbody (ea = 1, considerable CO2), the atmosphere is Ta = 255 K (as before) and the surface is Ts = 303 K (16 K warmer than actual value of 287 K). If ea = 1/2 (from less CO2), the atmosphere is too cold at Ta = 230 K and the surface is also too cold at Ts = 274 K. By adjusting ea to 0.76, we obtain the “correct” surface temperature, Ts = 287 K.
Multi-Layer Atmosphere. Next we divide the planetary atmosphere into n zones, layered vertically. By using several layers, the temperature gradient in each layer is reduced, smoothing the temperature profile to become more continuous. The thickness of a layer is such that almost all incident IR on a layer is just absorbed in that layer, which then radiates it upwards and downwards. Planets with small amounts of CO2 and H2O have less than one zone, while Venus has many zones. Due to lack of space, we leap to the answer:
T0 = [(1 – a)so/4s]1/4 and Ts = (n + 1)1/4T0, (18)
wheren is the number of IR absorption layers Earth has. Earth’s so = 1367 W/m2 and a = 0.3 gives T0 = 255 K, T1 = 303 K, T10 = 464 K, T20 = 546 K and T75= 753 K. The answer depends greatly on the amount of greenhouse gasses in the atmosphere. Earth's surface temperature of 287 K is somewhat colder than that for one full layer (n = 1) at 303 K. The number of layers for the Earth’s atmosphere is obtained by solving for n, giving
n= (Ts/T0)4 – 1 = (287 K/255 K)4 – 1 = 0.6. (19)
It is not surprising that Earth's atmosphere contains only 60% of an IR layer since O2 and N2 hardly absorb IR, leaving the task of IR absorption to trace amounts of CO2 and H2O. Venus, on the other hand, has a large temperature difference between the upper atmosphere at T0 = 229 K and the surface at Ts = 750 K. These temperatures give 74 IR layers for CO2 rich Venus!
Solar Variations. We might expect solar variations of 0.2% are possible since that is twice the present 11-year solar variation.The 0.2% variation gives a surface temperature variation of
ΔTs = Ts(Δs/so)/4 = (287 K)(2 x 10–3)/4 = 0.14 K. (20)
Correlation has been discovered between number of sunspots and surface temperature of Earth. However, for solar variations to explain climate change, there remains to be identified an additional solar heating mechanism beyond that already described. General circulation model calculations show extra heating in summer warms the stratosphere, strengthening easterly winds and changing wind patterns. However, the GCM changes predicted from solar variations are smaller than the observed changes. Other GCM calculations, which include interactive stratospheric chemistry with ozone, had more success in predicting an 11-year climate cycle. A theoretical link between solar variation and climate change needs a more active sun to emit considerably more ultraviolet. Extra UV would interact with ozone, raising stratosphere temperatures, but this would only raise the surface temperature at high latitudes by only a few tenths of a degree. Our calculation supports the IPCC findings that the contribution of solar variations to increased temperatures is not significant. Figure 3 in the paper by Judith Lean indicates that the cyclical amplitude of Earth’s surface temperature is about 0.1 K, so the solar variational effect is not significant.[iv] On the other hand, our calculations, the GCMs, and Arrhenius can explain the observed global temperature rises with the observed increases in greenhouse gases.
Conclusion: Earth is getting warmer. Basic atmospheric models clearly predict that additional greenhouse gasses will raise the temperature of Earth. To argue otherwise, one must prove a physical mechanism that gives a reasonable alternative cause of warming. This has not been done. Sunspot and temperature correlations do not prove causality.
David Hafemeister and Peter Schwartz
Physics Department
Cal Poly University, San Luis Obispo, CA 93405
dhafemei@calpoly.edu
[i] Intergovernmental Panel on Climate Change, Climate Change 2007: The Physical Basis, Cambridge University Press, New York (2007), http://www.ipcc.ch.
[ii] D. Hafemeister, Physics of Societal Issues: Calculations on National Security, Environment and Energy, Springer, New York, 2007.
[iii] N. Scafetta and B. West, “Is Climate Sensitive to Solar Variability,” Physics Today 61, 50–51 (2008).
[iv] J. Lean, “Living with a Variable Sun,” Physics Today 58, 32–38 (2005).
The Forum on Physics and Society is a place for discussion and disagreement on scientific and policy matters. Our newsletter publishes a combination of non- peer- reviewed technical articles, policy analyses, and opinion. All articles and editorials published in the newsletter solely represent the views of their authors and do not necessarily represent the views of the Forum Executive Committee.
