The Future of Automotive Lithium-ion Battery Recycling: Charting a Sustainable Course
Linda Gaines (lgaines@anl.gov), Argonne National Laboratory
INTRODUCTION
Recycling, per se, is not inherently good or bad (1). For materials like glass (2), the benefits are dubious and depend on factors like the shipping distance. There has also been some debate on the benefits of recycling primary alkaline batteries given the abundant and non-toxic nature of the components (3). For automotive batteries, however, the environmental benefits are clear, although they vary with battery type and recycling method. There are also potential economic benefits. If usable materials can be recovered, less raw material needs to be extracted from the limited supplies in the ground. Further, domestic recycling reduces the the raw materials imported from abroad, improving our balance of payments. In addition, significant environmental benefits can be obtained by recycling elements obtained from mining and processing ores (e.g., SOx emissions from smelting of sulfide ores to yield copper, nickel, and cobalt) (4) since the environmental effects of recycling are generally smaller than those from primary production. There are, of course, exceptions, such as recovering lithium from pyrometallurgical process slag. Recycling of materials also avoids processing costs for waste treatment. In addition, some spent batteries (5) are classified as hazardous waste, which increases transportation, treatment, and disposal costs, and requires additional effort to achieve regulatory compliance.
Lithium-ion batteries are starting to be used in significant quantities for automotive propulsion. Because these batteries are expected to last the life of the vehicle and may subsequently be used for utility energy storage, they will not be ending their useful lives in large numbers for on the order of 10 years. What steps can be taken to ensure that these spent Li-ion batteries are recycled at the end of their useful life. In an ideal system, these batteries would be sent for responsible recycling and not exported to Third World countries with less stringent environmental, health, and safety regulations. Methods are needed for safe and economical transport and processing of the spent batteries, as well as environmentally sound recycling practices. In addition, the recycled product needs to be of sufficient quality to find a market. Fortunately, recycling system for lead-acid and Nickel-Metal Hydride batteries are already in place and can provide lessons that can be applied to recycling Lithium-ion batteries.
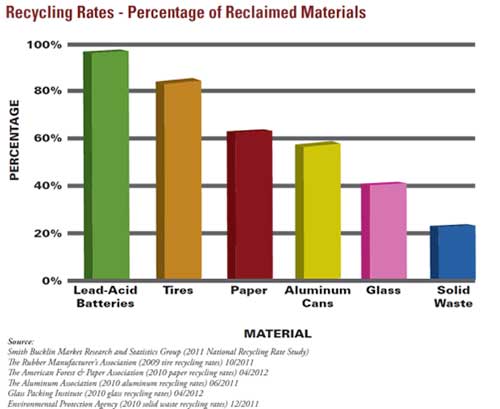
FIGURE 1: Batteries are the most recycled consumer product. (Courtesy of Battery Council International)
LEAD-ACID BATTERY EXAMPLE
Lead-acid batteries are recycled more than any other major consumer product, Figure 1. In the US, about 98% of lead-acid (Pb-acid) batteries are recycled (6). Lead-acid battery recycling is also working well in Europe and Japan. In countries like China, significant changes have recently been made to improve the recycling practices (7). To date, the model shown in Figure 2 has worked admirably for lead-acid battery recycling. Would some variation of this model work as well for other battery types?
COMPARISON OF AUTOMOTIVE BATTERY TYPES
Before examining that question, it is useful to first compare the physical and chemical structures of different types of automotive batteries; namely, the lead-acid batteries used for starting-lighting-ignition (SLI) commonly found under the hood of most cars, nickel-metal-hydride (Ni-MH) batteries used in hybrid vehicles, and Li-ion batteries used in plug-in and some hybrid vehicles. The latter two battery types are used primarily for propulsion and heating, ventilation, and air conditioning (HVAC). Conceptually and structurally, these battery types are similar but chemically quite different. Each consists of electrode (cathode and anode) active materials on grids or foils that serve as the current collectors, with an electrolyte that carries charge between the electrodes. These components are housed in an enclosure. The compositions of these components differ greatly among the battery types, Table 1.
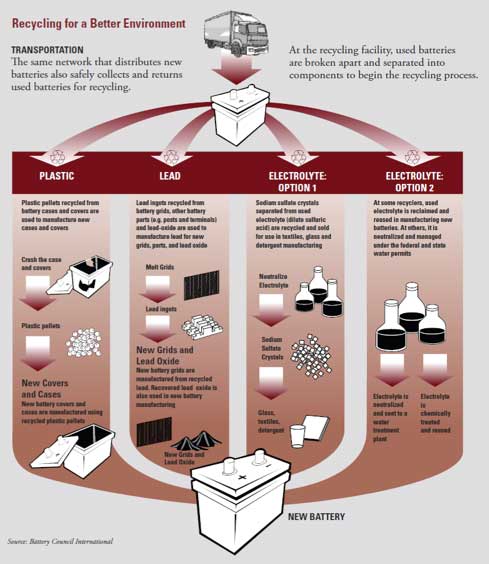
FIGURE 2: Simple processes are used to recycle lead-acid batteries. (Courtesy of Battery Council International)
TABLE 1: Comparison of cell materials
| Cell component/battery type | Pb-acid | Ni-MH | Li-ion |
|---|---|---|---|
| Cathode | PbO2 | Ni(OH)2 | LiMO2 |
| Cathode plate/foil | Pb | Ni foam | Al |
| Anode | Pb | MH (AB5) | graphite |
| Anode plate/foil | Pb | Ni-plated steel | Cu |
| Electrolyte | H2SO4 | KOH | Organic solvent + LiPF6 |
| Separator | PE or PVC w/silica | polyolefin | PE/PP |
| Cell case | PP | Stainless steel | Varies (metal or laminate) |
PE = polyethylene; PVC = polyvinyl chloride; PP = polypropylene.
The more diverse the battery materials, the more complex the recycling. For Pb-acid batteries, all of the internal components contain lead, which makes up about 60% of the battery mass. Except for electrical connectors, which can be removed when the cells are opened, no other metal is present. As a result, no separation processes are required. Because nickel dominates the Ni-MH battery, it can be the focus of recycling. In addition, the nickel and steel alloys that results from current recycling processes are a valuable input to stainless steel manufacturing, making recycling of these batteries economical today. The many different materials in a typical Li-ion battery complicate recycling.
Lead-Acid Battery Recycling
Disposal of Pb-acid batteries is illegal in most states and many states, as an incentive for consumers to return their batteries, require a monetary deposit. Most Pb-acid batteries are collected when new ones are purchased (the dealers are required to accept them and are paid for their trouble). Additionally, as required by law, batteries are stripped from vehicles that have gone out of service and are about to be shredded. Regulations concerning transportation and processing of batteries are in place and widely known.
Lead-acid battery are recycled by a simple process, Figure 2. First, the battery case is broken open and the sulfuric acid electrolyte is drained out and collected. The plates and connectors, Figure 3, can be removed from the case at this point and recovered whole. Alternatively, the drained battery can be sent to a hammer-mill for size reduction, and the plastic and lead separated by a simple sink-float device. The recovered lead is re-smelted and purified to make new battery components, which avoids the sulfur emissions from primary lead production with the lead emissions from secondary smelting being tightly regulated by the Environmental Protection Agency (8). The plastic is melted and molded into new cases. The acid can be neutralized or processed to sulfate salts for various uses, such as the manufacture of soap.
The recycling operation is profitable because recycled lead (taken back to its elemental form and purified) is known to be of high quality and there is little incentive to export to places with less stringent regulations, although some batteries do find their way to Mexico (9). Some battery manufacturers prefer new over recycled lead.
A key reason for the success of lead-acid battery recycling is that essentially all of the manufacturers use the same raw materials: lead, lead oxide, and sulfuric acid in a polypropylene case. Because the battery design is similar for the manufacturers, automated technology can be used for battery disassembly. Further, lead-acid recycling works well because it is profitable, it is illegal to dispose of the batteries without recycling, the battery chemistry does not require segregation, and the recycling process is simple.
Nickel-Metal Hydride Battery Recycling
Large-format Ni-MH batteries have been used in hybrid vehicles for long enough that some now require disposition by either reuse or recycling and a recycling system is already in place because consumer batteries from smaller devices, such as power tools, have been recycled commercially for many years, Figure 4. However, not all of the battery materials are recovered as high-value products. The nickel and iron are recovered by rotary hearth and electric-arc furnaces as ferronickel to feed stainless steel production. Because this is a high-value product with a huge market, there is no need to separate the nickel from the iron. However, this technique loses rare earths in the metal hydride to the slag, which is used as roadbed aggregate in place of gravel (10). The increasing demand for rare earths in batteries, motors, and other components of vehicles and wind turbines, coupled with China’s policies to restrict exports so that they meet their own demand, has provided a significant economic incentive to recover these metals during recycling.
Nickel-metal hydride batteries have a similar chemistry (AB5), although the exact mix of rare earth elements may vary slightly and pack configurations differ. For that reason, differentiation among Ni-MH batteries is not needed. Several companies have announced programs to recover the rare earths from Ni-MH batteries. Umicore, in Belgium, recovers nickel and has an agreement with Solvay (formerly Rhodia) to recover rare earths from slag and (11). Retriev Technologies (formerly Toxco) has a plant under construction in Ohio, partially funded by Recovery Act funds received in 2009, and plans to recover rare earths when its first processing line is completed. Honda has an agreement with Japan Metals and Chemicals(12) to recycle its Ni-MH batteries. In Australia, Toyota offers a $100 rebate when a Prius battery pack is returned, and a discount on a replacement (13). In sum, Ni-MH batteries seem to be on track for successful recycling.

FIGURE 3: Lead-acid battery cases and plates after separation.

FIGURE 4: Hand-sorting of Ni-MH consumer batteries (Courtesy of Kinsbursky Brothers, Inc.)
Lithium-Ion Battery Recycling
Several factors contribute to making Li-ion battery recycling more complicated than Pb-acid or Ni-MH recycling. First, as shown in Table 1, Li-ion batteries have a wider variety of materials in each cell. The active materials are in the form of powder, coated onto metal foil, and these different materials must be separated from each other during recycling. Within the cells, the chemical compositions of the active materials, especially the cathode, vary with manufacturer and battery function, are changing, and may never standardize. The most common cathode material for the batteries in consumer electronics is LiCoO2 (LCO), but various combinations of Ni, Mn, and Al can replace some or all of the cobalt to optimize performance while lowering raw material cost, which is key for automotive batteries. Another promising cathode material is LiFePO4 (LFP). Most manufacturers use some form of graphite for the anode, but silicon is also used and other materials/mixtures are being studied. Lead-acid batteries have a relatively small number of large lead plates packed together in a single plastic case, while a Li-ion pack is likely to have 100 or more individual cells (~5000 for a Tesla electric vehicle), which are connected into modules and assembled into a pack with control circuitry and thermal management systems attached to each cell, Figure 5. These components could possibly be recovered intact or may contain valuable materials that could provide some economic incentive for recycling.


FIGURE 5: Lead-acid battery and Li-ion pack
Lead-acid batteries are small and easily removed from their location under the hood, while the larger, more complex Li-ion packs vary in shape and location in the vehicle. As a result, removal may be more difficult. However, if removal takes a professional, fewer people need to be trained on proper handling and separation. Further, if Li-ion batteries last for the life of the vehicle, they will all end up in scrapyards or at auto dealers, which are both sufficiently large enterprises to facilitate collection. Vehicle batteries being subsequently used for stationary energy storage could also be collected from utilities after this second use.
At present, there are no regulations regarding recycling of large-format Li-ion batteries. This condition might be thought to be good for recyclers, who would face no restrictions in process design. However, they face the possibility that restrictive regulations could later be imposed. Therefore, processes must be designed to be compliant with anticipated regulations. In addition, the technology is still evolving and recycling processes designed for a specific design or chemistry could become irrelevant.
Automotive Li-ion batteries have only been in commercial use for about 5 years, and it will take some time until they are used in large volumes. Further, their long product life (ideally, the life of the car), means that not nearly enough batteries have reached the end of their lives to support large-scale recycling plants. However, consumer lithium batteries and processing scrap are available and could supply feedstock for a fledgling recycling industry for automotive Li-ion batteries. Several recycling methods have been proposed, each with its advantages and disadvantages, as discussed below and described in Ref. 14.
Pyrometallurgical Recycling (Smelting)
After dismantling the lithium-ion batteries to the module level, they are fed to a high-temperature shaft furnace with a slag-forming agent that typically includes limestone, sand, and slag. The electrolyte and plastics burn to supply some of the energy for the smelting, and valuable metals are reduced to an alloy of copper, cobalt, nickel, and iron, which are then recovered by leaching. The resulting slag contains lithium, aluminum, silicon, calcium, iron, and any manganese that was present in the cathode material. Recycling aluminum or lithium from the slag is not economical or energy efficient and gas cleanup steps are necessary to avoid the release of potentially toxic by-products. This process is operating commercially and is economical for batteries with cathode materials containing cobalt and nickel but not for newer designs with manganese spinel or LFP cathodes, Table 2.
TABLE 2: Recovery of cathode material maximizes product value
| Cathode | Price of Constituents ($/lb) | Price of Cathode ($/lb) |
|---|---|---|
| LiCoO2 | 8.30 | 12-16 |
| LiNi1/3Co1/3Mn1/3O2 | 4.90 | 10-13 |
| LiMnO2 | 1.70 | 4.50 |
| LiFePO4 | 0.70 | 9 |
Intermediate Recycling Process
In this process, commercially used in Canada, batteries undergo size reduction in a hammer-mill and a shaker table separates mixed plastics and metals. Filtering of the aqueous stream leaving the hammer-mill yields mixed metal oxides, carbon and a liquid stream which is dewatered to some extent. It is then mixed with soda ash to precipitate Li2CO3, which is subsequently filtered from the solution and sold. The metals (including the Al) can be separated and sent for recycling. However, as with pyrometallurgical recycling, the process is only economical if cobalt and/or nickel are contained in the cathode.
Direct Recycling
This bench-scale physical process, which has been demonstrated for several cathode materials, recovers battery materials for reinsertion into the battery supply chain with little or no additional processing. Breached discharged cells are placed in a container to which CO2 is added, and the temperature and pressure are raised to bring CO2 above its critical point. The supercritical carbon dioxide extracts the electrolyte (ethyl methyl carbonate, diethyl carbonate, and LiPF6) from the cells and is removed. The electrolyte separates from the gaseous CO2 and, after further processing, can be recycled for use in batteries. The cells, devoid of electrolyte, undergo pulverization or other size-reduction steps, possibly in the absence of water or oxygen to avoid contamination of materials. Subsequently, the cell components are separated through techniques that exploit differences in electronic conductivity, density, or other properties. Cathode materials may need to undergo re-lithiation prior to reuse in batteries.
This process has the advantage that almost all battery components, including aluminum but excluding separators, are recovered and can be reused after further processing. Most important, the cathode materials constitute a potentially valuable product from direct recycling, regardless of cathode type. There is some question, however, on whether the recovered material will perform as well as virgin material, which could have implications for battery power and lifetime. As such, manufacturers may be reluctant to purchase recycled compounds. Manufacturers of products with tight quality standards have historically been reluctant to purchase recycled materials because of performance concerns (15). Product quality will depend on having a known, uniform input stream; mixing cathode materials is likely to reduce the recycled product value.
Discussion
Several factors could help promote Li-ion battery recycling. Packs are large and recognizable. They will be removed from end-of-life vehicles if there is an economic incentive or a regulatory imperative. They will be labeled to enable identification for proper routing. The recovered batteries could be returned to the original manufacturers. However, while these manufacturers would know what the batteries are composed of, they might not want to be in the recycling business and they would be required to process recycled compounds that could be obsolete 10 years or more after initial production.
Several developments would facilitate making Li-ion battery recycling economically viable:
- Separation technology that enables processing different chemistries, recycling processes for each cell chemistry, or technology that produces valuable products from a mixed stream;
- Methods for separation of cathode materials after initial processing;
- Greater recycling process flexibility or standardization of battery materials and designs; and
- Assurance that regulations will not impede recycling.
Initial battery manufacturers can promote eventual recycling using design for recycling, including the following steps: inclusion of labels or other distinguishing features, use of a minimum number of different materials, standardization of formats and materials, avoidance of toxic materials (cadmium, arsenic, mercury, halogens, etc.), and designs that allow easy separation of parts, including a separable cooling system, reversible joining (i.e. nuts and bolts instead of welds), and avoidance of potting compounds to hold cells in place. Of course, these design changes cannot be made at the cost of any reduction in performance or safety during the battery’s useful life. A committee of the U.S. Advanced Battery Consortium (USABC) is working on design-for-recycling guidelines for U.S. auto manufacturers (16).
Work is underway to address all of the roadblocks to Li-ion battery recycling within the 10 years or so before large numbers of large-format automotive batteries are ready for final disposition.
A PROBLEM IN THE MODEL RECYCLING SYSTEM
Recent events where Li-ion batteries have been included the lead-acid battery input stream at secondary lead smelters have resulted in fires and explosions (17) and have identified issues with the system on which future Li-ion recycling can be modeled. This contamination of the input stream may be the result of honest mistakes due to the fact that many current Li-ion batteries are indistinguishable from lead-acid batteries. It may also be a consequence of recyclers paying for their desired input material (Pb-acid batteries) and charging for disposition of other less-desired input material, resulting in contamination to avoid these fees. Regardless of the reason, such events pose a serious danger and must be prevented. In practice, however, it is difficult to detect due to the similar structure of the batteries and that batteries are often delivered to recyclers in huge loads (over 3,000/hour– up to 70,000/day). This problem could be reduced if there were a profitable outlet for the recycling of Li-ion batteries or if all Li-ion batteries could be recycled to high-value products, Table 2. At the present time, however, Li-ion manufacturers are moving toward cheaper materials, which exacerbates the problem. Any model system for battery recycling will need to avoid cross-contamination. This issue is being addressed in the U.S. by the Society of Automotive Engineers and in Europe by EUROBAT. Both groups have active working groups attempting to better define and find solutions to the problems of cross-contamination of battery types in recycling streams (18,19).
While segregation systems for large-format batteries are needed, the optimum separation point in the recycling chain is unclear, and rescreening might still be necessary before final processing. The screening could be based on density differences (Li-ion batteries are likely much lighter than Pb-acid batteries) but careful separation is likely to increase recycling costs, which would likely be borne by consumers. Separation could be facilitated if manufacturers labeled battery components by means of bar codes, RFID chips, or delegated paint color or type (e.g. visible under black light). It would also be helpful to have clear incentives for good recycling practices and penalties for poor practices.
VISION OF IDEAL FUTURE SYSTEM
Ideally, the search for the best battery chemistries and designs will result in a few designs that satisfy everyone’s requirements, and the batteries of a given type would be made as uniform as possible. Further, those that could be recycled together would have at least one distinguishable, common feature. Conversely, different battery types would look different and have a least one feature that differentiates them from batteries to be recycled in a different way. This would require that batteries be designed with recycling in mind. There would need to be an easy way to route these spent batteries to the appropriate recycling facilities in a safe and legal manner at the conclusion of their useful lives and regulations that assure safe transport and handling while discouraging cross-contamination would need to be in place. Sorting/routing could be immediate, via a transfer station, or take place within a unified recycling facility. Separate streams would be processed to produce valuable, high-purity materials that could be reused in batteries or other high-valued products. An alternative to separate processing would require process development to enable production of a valuable product from a mixed stream (or product separation into valuable streams). In either case, strict industry standards would be needed to ensure that recycled products meet the same high quality standards as virgin materials to facilitate their reuse.
Accomplishment of this future vision before large numbers of automotive propulsion batteries have reached the end of their useful lives requires research and planning to continue over the next 10 years or so. It is a daunting task, but it can be accomplished if there is a broad commitment from industry and government.
REFERENCES
1. Gaines, L. To Recycle or Not to Recycle: That Is the Question — Insights from Life-Cycle Analysis. MRS Bulletin, Vol. 37, 2012, pp.333-338
2. Gaines, L., and M. Mintz. Energy Implications of Glass-Container Recycling. Argonne National Laboratory Report ANL/ESD-18, 1993.
3. Babiak, M.,and M. Boolish. Primary Battery Collection and Recycling: A Manufacturer’s Perspective. National Electrical Manufacturers Association (NEMA), 13th International Battery Materials Recycling Seminar & Exhibit, Ft. Lauderdale, FL, March 16-18, 2009.
4. Dunn, J.L. Gaines, J. Sullivan, and M.Q. Wang.The Impact of Recycling on Cradle-to-Gate Energy Consumption and Greenhouse Gas Emissions of Automotive Lithium-Ion Batteries. Environmental Science and Technology, Vol. 46, 2012, pp. 12704-12710.
5. Lithium-ion batteries are classified as Class 9 miscellaneous hazardous materials, and lead-acid batteries are listed as Class 8 corrosive hazardous materials under 40 CFR 173.21(c), see PRBA: The Rechargeable Battery Association. Information on Batteries in Transport. Accessed July 10, 2014.
6. Battery Council International. Battery Recycling. http://batterycouncil.org/?page=Battery_Recycling. Accessed July 9, 2014.
7. McDonald, J. China Recycling Cleanup Jolts Global Industry. Associated Press, October 3, 2013. https://www.usnews.com/news/world/articles/2013/10/03/china-recycling-cleanup-jolts-global-industry. Accessed July 9, 2014.
8. Environmental Protection Agency. Rule and Implementation Information for Secondary Lead Smelters. http://www.epa.gov/ttn/atw/lead2nd/lead2pg.html. Accessed July 9, 2014.
9. Rosenthal, E. Lead from Old U.S. Batteries Sent to Mexico Raises Risks. New York Times, December 8, 2011. http://www.nytimes.com/2011/12/09/science/earth/recycled-battery-lead-puts-mexicans-in-danger.html?pagewanted=all&_r=0. Accessed July 9, 2014.
10. Gaines, L., M. Singh, and D. Elcock. Nickel-Metal Hydride Batteries: Energy Use and Emissions from Production and Recycling. SAE Future Car Congress, Arlington, VA, June 2002.
11. Umicore and Rhodia Develop Unique Rare Earth Recycling Process for Rechargeable Batteries. Press Release CP-2011-18-R, June 6, 2011. http://www.umicore.com/investorrelations/en/newsPublications/pressReleases/2011/show_REErecyclingEN.pdf. Accessed July 10, 2014.
12. Honda to Reuse Rare Earth Metals Contained in Used Parts. Press Release, April 17, 2012. http://world.honda.com/news/2012/c120417eng.html. Accessed July 10, 2014.
13. Hybrid Battery Recycling Program. http://www.toyota.com.au/hybrid-battery-recycling. Accessed July 10, 2014.
14. Dunn, J.B., L. Gaines, M. Barnes, J. Sullivan, and M. Wang. Material and Energy Flows in the Materials Production, Assembly, and End of Life Stages of the Automotive Lithium Ion Battery Life Cycle. Argonne National Laboratory Report ANL/ESD/12-3, June 2012.
15. Gaines, L., and A. Wolsky. Discarded Tires: Energy Conservation through Alternative Uses. Argonne National Laboratory Report ANL/CNSV-5, December 1979.
16. 2014 Recommended Practice for Recycling of xEV Electrochemical Energy Storage Systems. United States Advanced Battery Consortium Report, draft, Spring 2014. Accessed July 2014.
17. ILA/ABR Survey Analysis. International Lead Association, April 2014.
18. Automotive Battery Recycing Identification and Cross-Contamination Prevention — Recommended Practice J3071, Society of Automotive Engineers Battery Recycling Working Group, draft, July 2014.
19. Battery Recycling Streams Working Group Conference Call Minutes, Association of European Automotive and Industrial Battery Manufacturers, May 2014.
These contributions have not been peer-refereed. They represent solely the view(s) of the author(s) and not necessarily the view of APS.
