Propagating Hexane Flame
Philipp A. Boettcher
Brian Ventura
Joseph E. Shepherd
California Institute of Technology
Pasadena, California
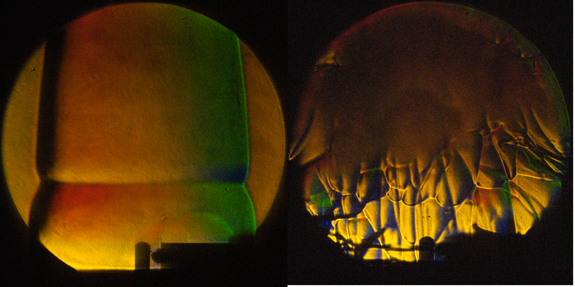
A flame, ignited by a glow plug at 650 degrees Celsius, is propagating in a gaseous mixture of hexane in air and developing instabilities.
In a mixture of hexane (4.32 %) and air containing an excess amount of the fuel hexane, a flame is ignited at the tip of the glow plug seen at the bottom of the image. The flame then propagates upwards and outwards and is captured in these image using color schlieren visualization, which allows us to see gradients in the density resulting from increase in temperature from the combustion. Additionally, we can observe a horizontal line across the flame, which is the initial instability resulting from the difference in flame speed in the hot plume above the glow plug and the much colder surrounding gas. The instability grows and forms a cellular flame as shown in the second photograph from the same experiment at a later time.
Ignition temperature and behavior of the flame propagation are important characteristics in safety analysis. In industrial and transportation applications combustible mixtures may come in contact with hot surfaces, simulated here by the glow plug, and ignite. The development of instabilities creates the wrinkled flame we see in the second frame, which has a larger surface area than a smooth flame. This increase in the flame area leads to acceleration of the flame. The flame speed is a factor in the peak pressure rise and thus potential structural damage resulting from an ignition event.
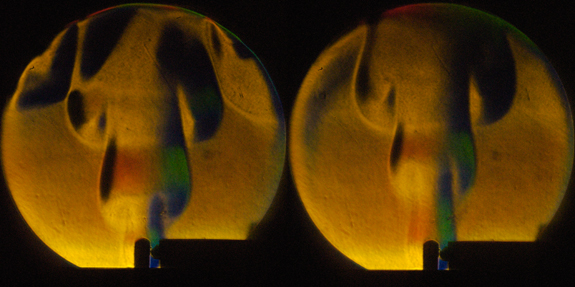
Puffing Hexane Flame: A gaseous mixture of hexane in air is consumed by a puffing flame over 7 seconds.
A homogeneous mixture of gaseous hexane (6.48 %) in air is heated locally by a glow plug until the mixture ignites. Unlike mixtures with lower concentrations of hexane this flame does not propagate outward and consume the reactants. The flame is instead swept upward by buoyancy and the reactants immediately following in its wake ignite. The two images show snapshots of this cyclic behavior which has a frequency of 12 Hz for this mixture. This behavior only ceases when the hot products of the reaction, which accumulate the top, fill up the 2 liter vessel up to the glow plug and prevent reactant to come in contact with the hot surface. Puffing behavior is generally associated with situations where fuel and air are not mixed beforehand such as a fires above a pool of liquid fuel [1].
To our knowledge, this is the first observation of a cyclic flames in homogeneous mixtures of gaseous fuel and air from a continuous hot surface. The most common situation is a single flame propagating through the mixture and consuming the reactants. The pressure rise seen here is very mild in comparison to that created by a single flame propagating through the mixture.
[1] Cetegen, BM and TA Ahemd. Experiments on the periodic instability of buoyant plumes and pool fires. Combustion and Flame 93, 1-2: 157-184, 1993.
This work was funded by The Boeing Company under Strategic Research and Development Research Agreement CT-BA-GTA-1.
References
Philipp A. Boettcher, B. Ventura , G. Blanquart, and J. E. Shepherd. "Hot Surface Ignition of Hydrocarbons in Air - A Comparison of Experimental and Computational Results." Eighth International Symposium on Hazards, Prevention, and Mitigation of Industrial Explosions (8th ISHPMIE). Manuscript under review.
Reporters and Editors
Reporters may freely use this image. Credit: P. A. Boettcher, B. Ventura, and J. E. Shepherd, California Institute of Technology (2010).
