Preferential Condensation
K. K. Varanasi
A. T. Paxson
Massachusetts Institute of Technology
Cambridge, Massachusetts
T. Deng
M. Hsu
N. Bhate
GE Global Research
Niskayuna, New York
Superhydrophobic surfaces are extremely water repellent, which makes them valuable for applications in energy conversion and water desalination. Some examples of superhydrophobic surfaces can be found in nature, such as the leaf of a Lotus plant and the skin of a Brazilian pygmy gecko.
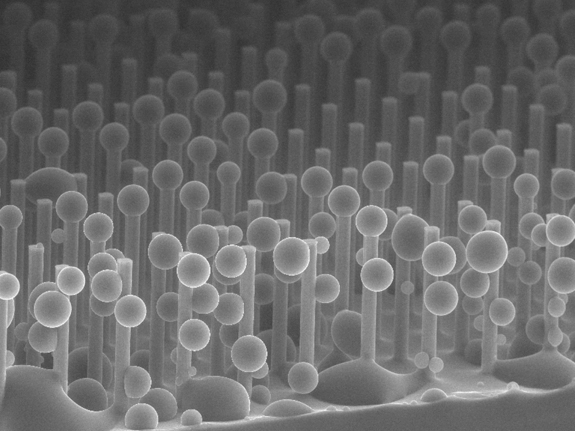
Figure A
In order to shed water so easily, these surfaces have multitudes of sharp microscopic bumps that support a droplet, as if resting on a bed of nails. If a water drop is able to sink into the valleys between these bumps, it becomes stuck. This becomes a big problem during condensation onto these superhydrophobic surfaces. Usually, microscopic water droplets condense randomly (Figure A) and begin growing in the valleys between the bumps, so by the time it grows into a large drop, it is strongly stuck to the surface (Figure B).
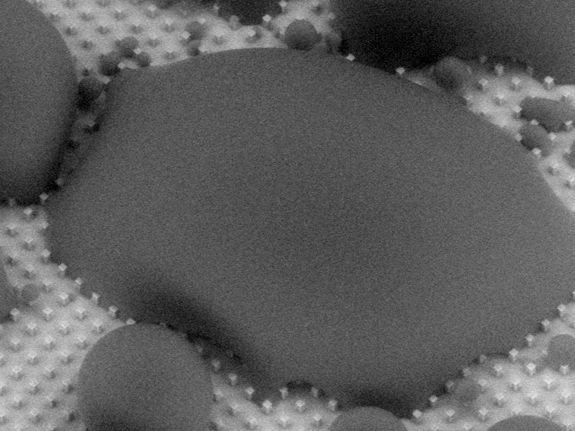
Figure B
Namib beetles have developed a strategy to overcome this problem in order to drink water in the desert. Their mostly waxy bodies are patterned with tiny wax-free patches. Condensation occurs only at these patches, so when the drops grow larger, they are only stuck at very few places and can roll off into the beetle's mouth.
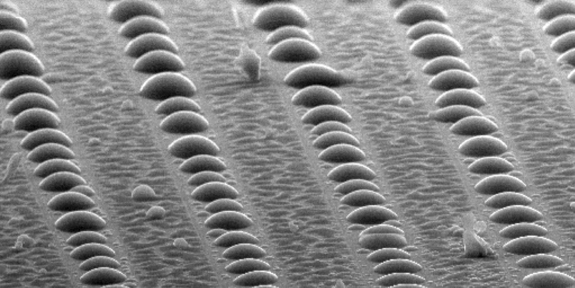
Figure C
Similarly, if we pattern a surface with hydrophilic (water-loving) and hydrophobic (water-hating) regions, preferential condensation occurs on the hydrophilic regions (Figure C). By placing these hydrophilic regions on the tops of hydrophobic bumps, we can preferentially condense on bump tops, prevent microscopic drops from condensing between the bumps (Figure. D) and overcome condensation-related limitations of superhydrophobic surfaces.
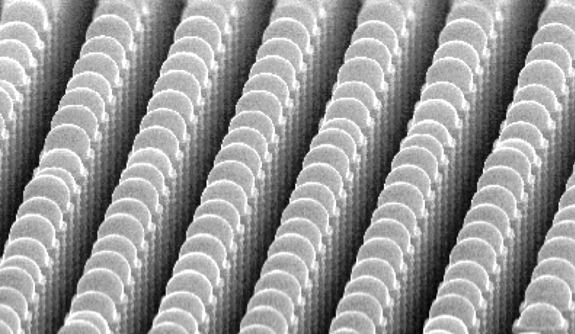
Figure D
The research is partially supported by General Electric and the MIT Energy Initiative.
References
[1] Quere, D (2005) Non-sticking drops, Rep. Prog. Phys. 68:2495-2532
[2] Varanasi, K. K., Hsu M., Bhate N., Yang W., Deng T. (2009) Spatial control in the heterogeneous nucleation of water vapor, APL, 95(9):094101-094101-3
[3] Parker, A. R. and Lawrence, C. R. (2001) Water capture by a desert beetle, Nature, 414:33-34
Reporters and Editors
Reporters may freely use these images. Credit: K. K. Varanasi, A. T. Paxson, T. Deng, M. Hsu, N. Bhate (2010).
